
Case study
Unifying & Enabling Teams Through a Streamlined Workflow
Client
Shield Therapeutics
industry
Life Sciences
Services
Phase 1-3 Build
UX & UI Design
Legal, Medical &
Regulatory Submissions
year
2021
Introduction
Shield Therapeutics created ACCRUFeR®, a stable, non-salt-based oral therapy for adults with iron deficiency, with or without anemia. For their FDA-approved launch, they partnered with Motionstrand to bring their new marketing campaign to life, leveraging our digital expertise to ensure a successful and impactful online presence.
Our goal
Motionstrand collaborated with Shield Therapeutics to create a seamless digital experience for the FDA-approved launch of ACCRUFeR®. Our creative and development teams worked with customer service to design all assets, managing the legal submission process through Veeva Vault to ensure an on-time launch.
Phase 1-3 Build
Setting the foundation for ACCRUFeR®
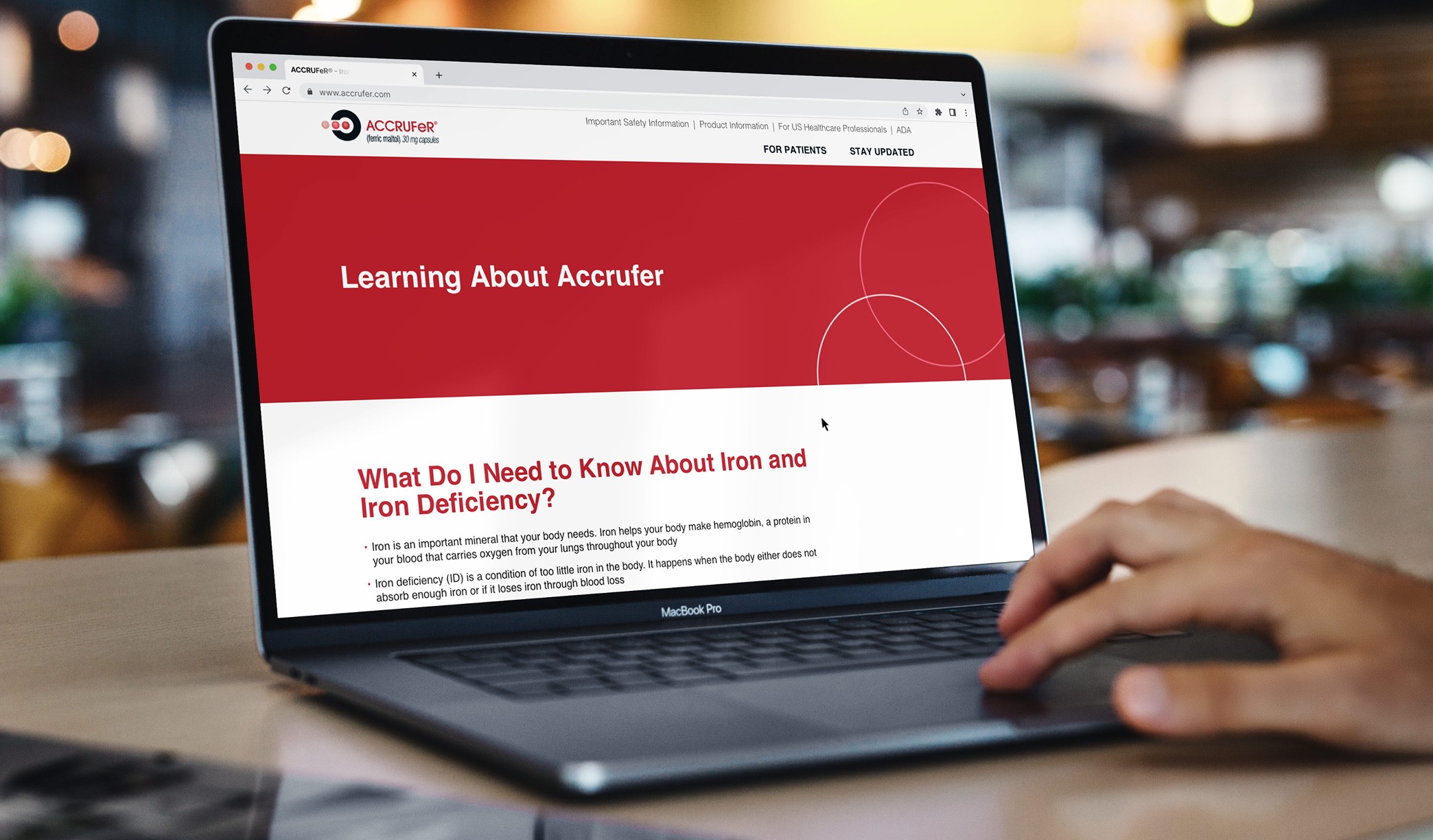
Motionstrand's priority was developing a Phase 1 website to introduce the ACCRUFeR® brand to healthcare providers. This initial site provided essential "coming soon" information while meeting all regulatory requirements. We designed every screen and interactive element for the Veeva Vault submission, ensuring compliance. Once approved, we moved into full development, secured legal clearance, and successfully launched the site.


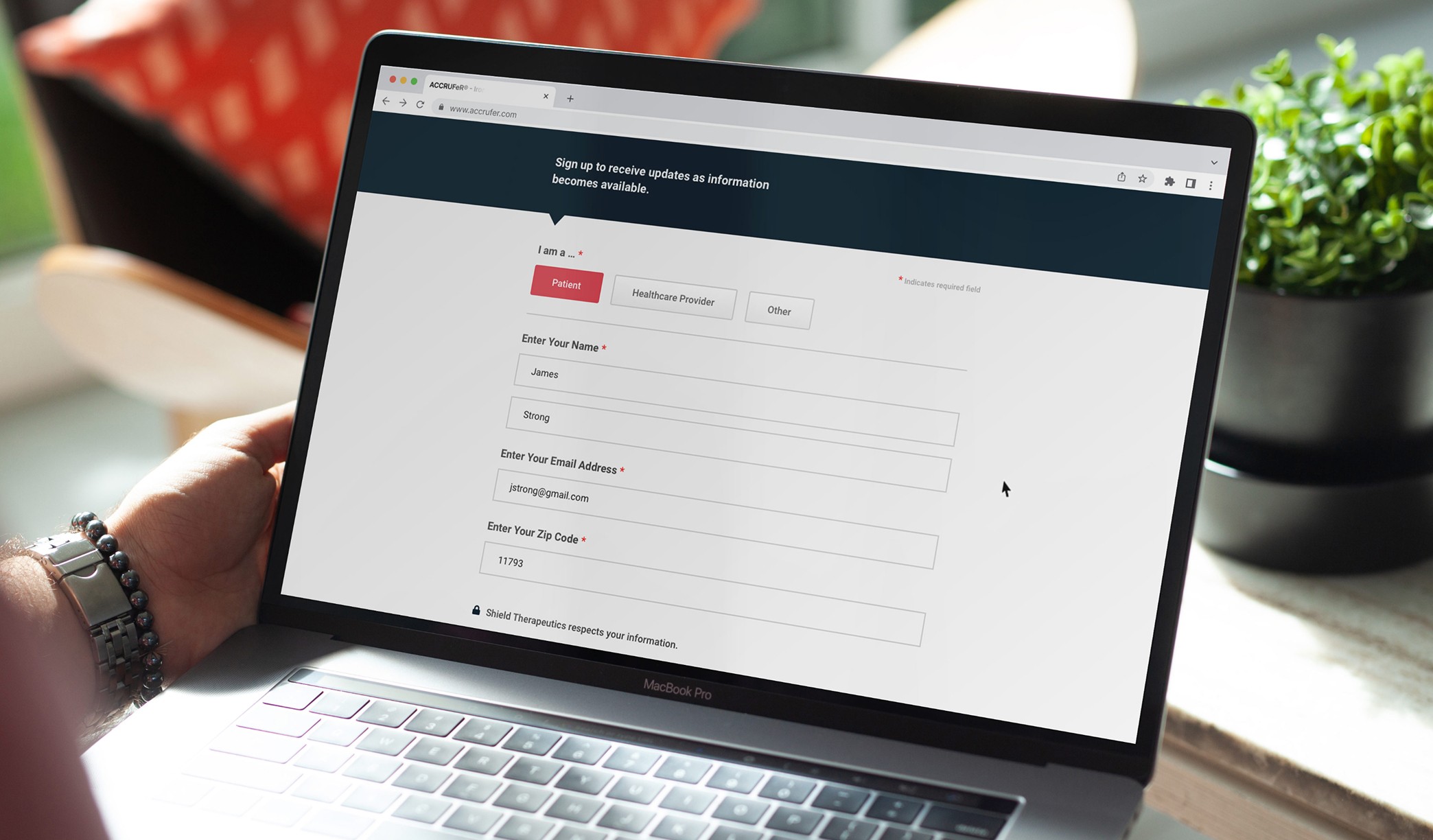
UX & UI Design
Crafting a cohesive digital experience
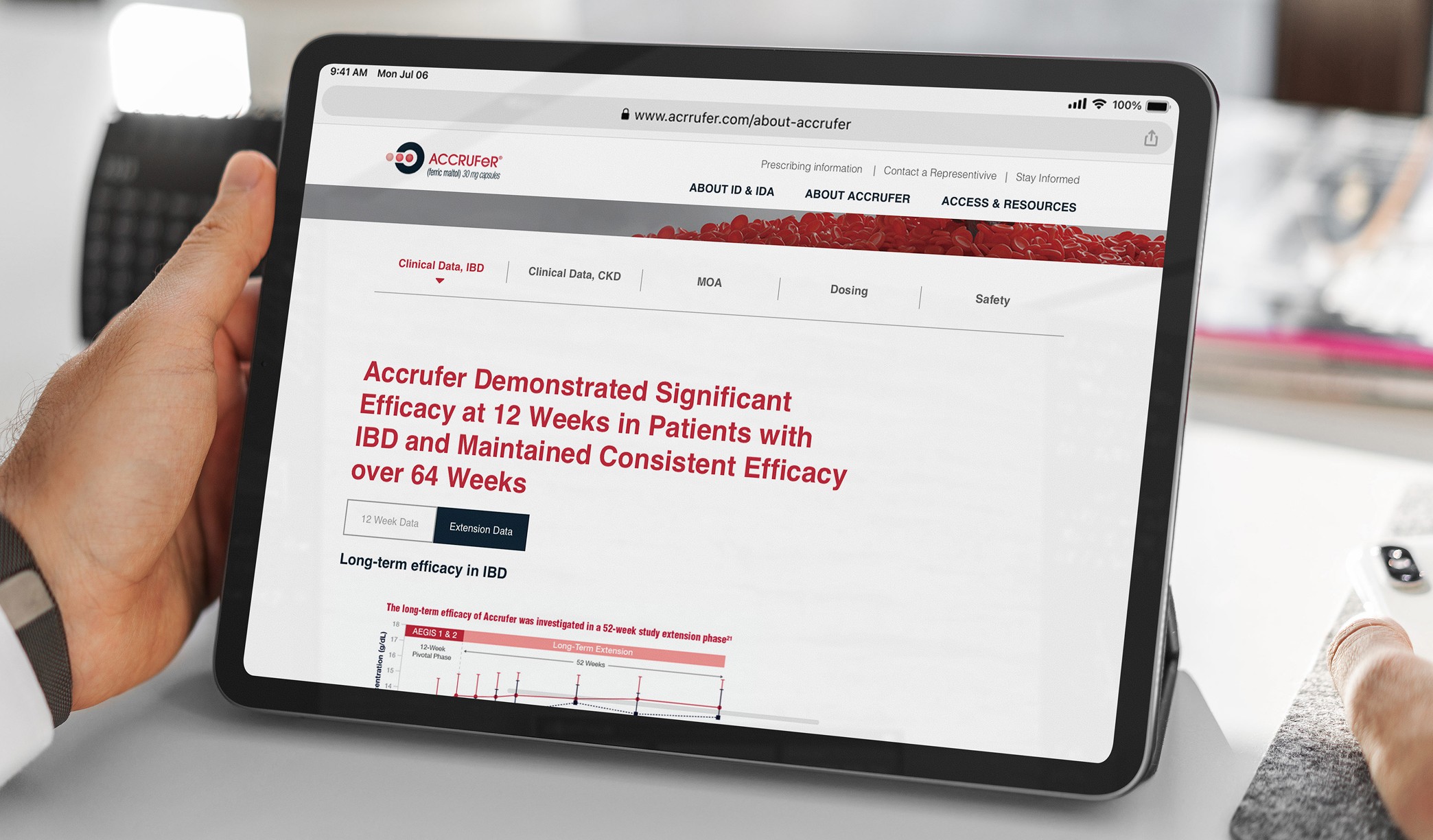
Motionstrand collaborated with ACCRUFeR®'s Agency of Record for the second phase to build fully branded DTC and HCP websites for the official launch. With strict deadlines in place, we started by creating wireframes to refine the user experience and interactions. The UI design was crafted to align seamlessly with the AOR's marketing materials, ensuring brand consistency. Our team then leveraged its deep expertise in Drupal development to deliver a scalable, regulatory-compliant digital presence.
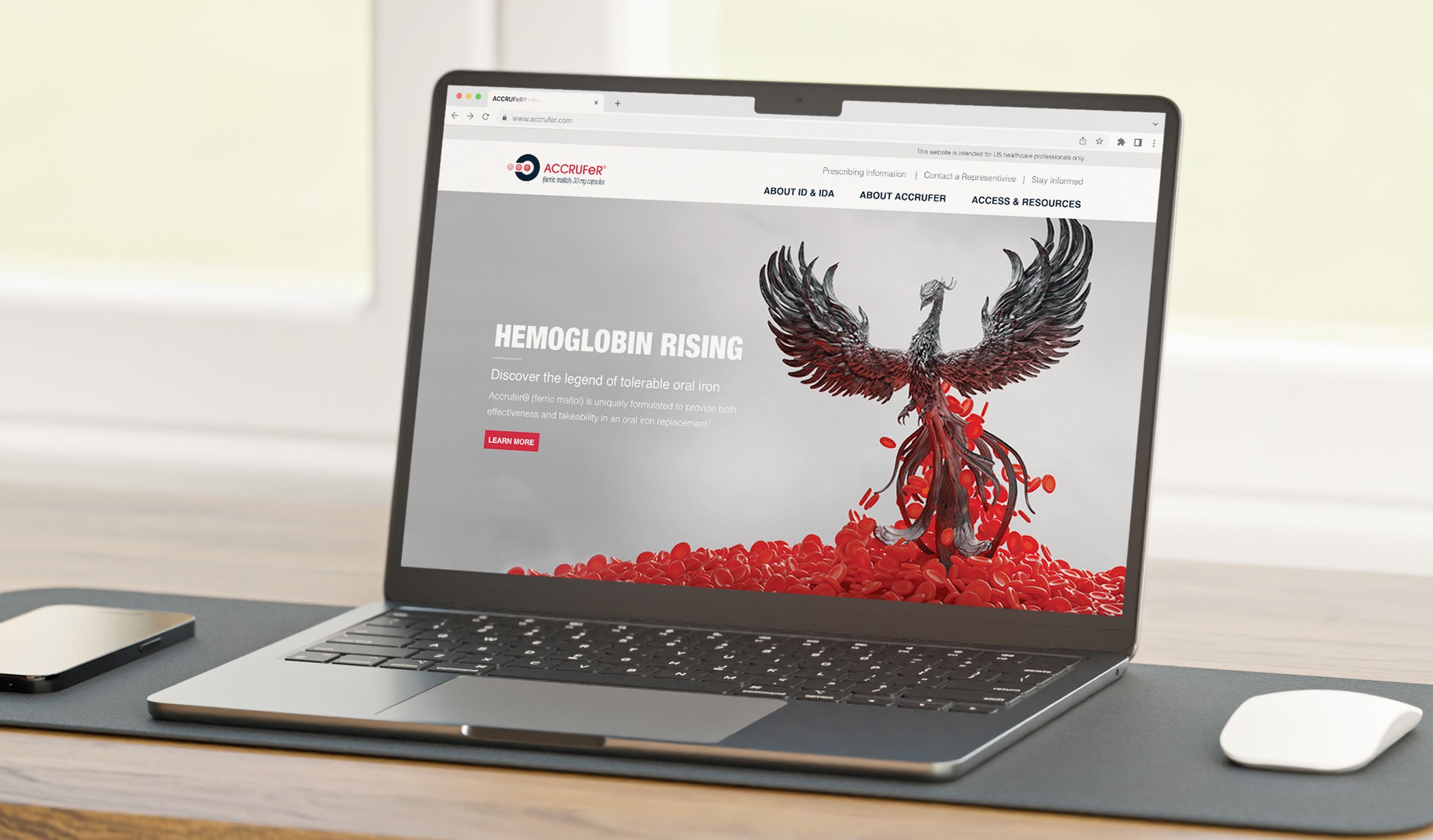
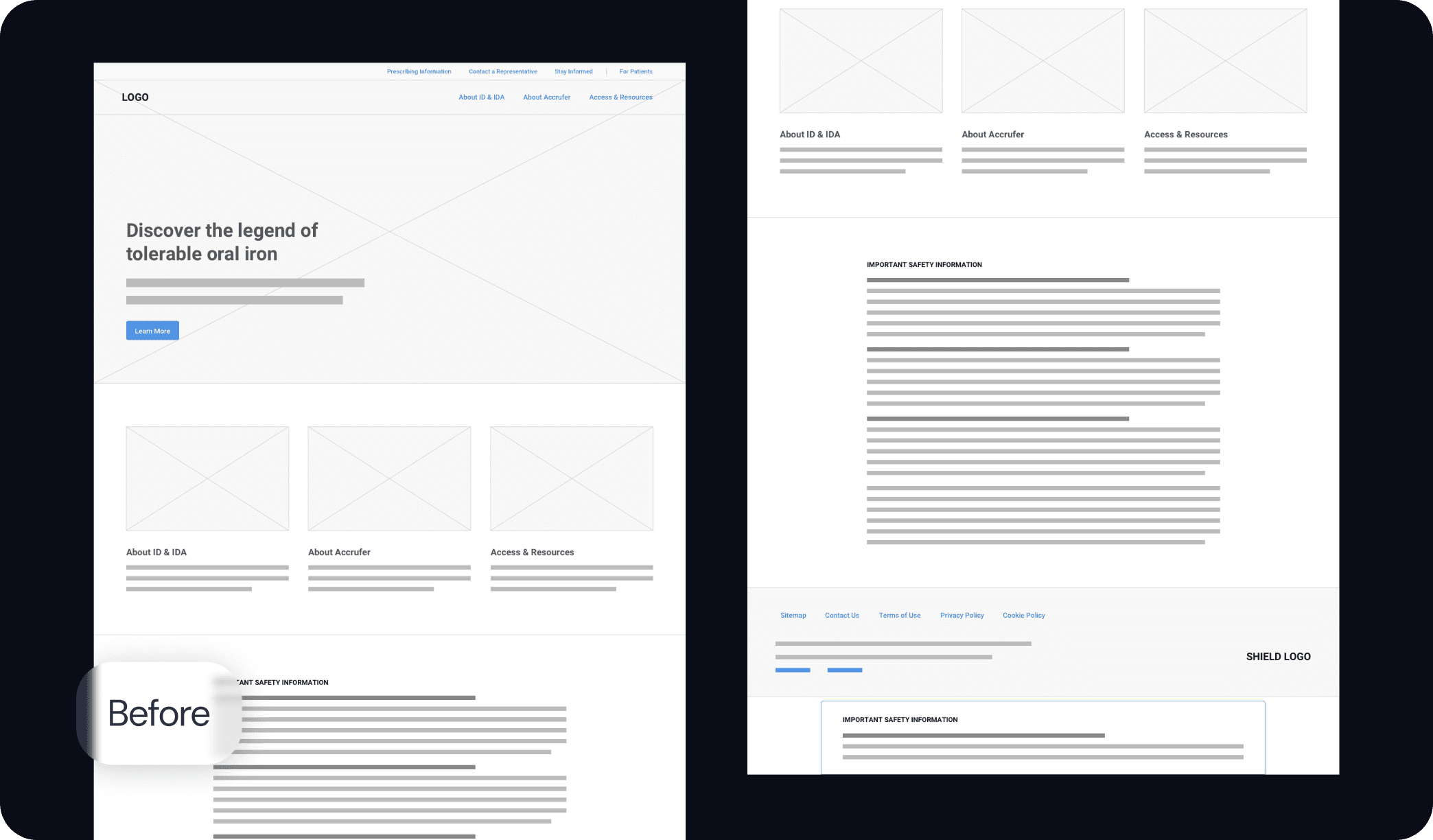
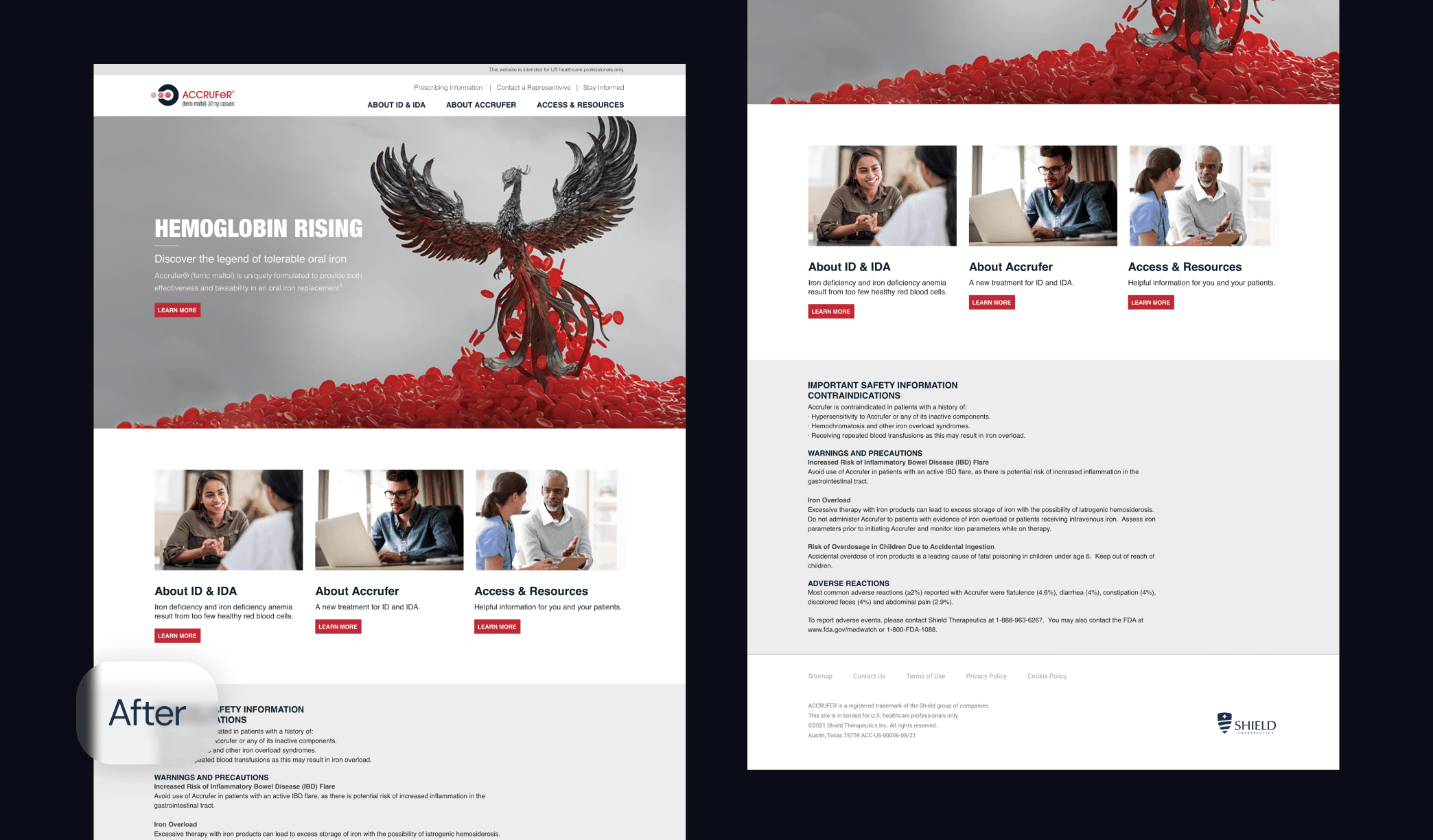
Legal, Medical, & Regulatory Submissions
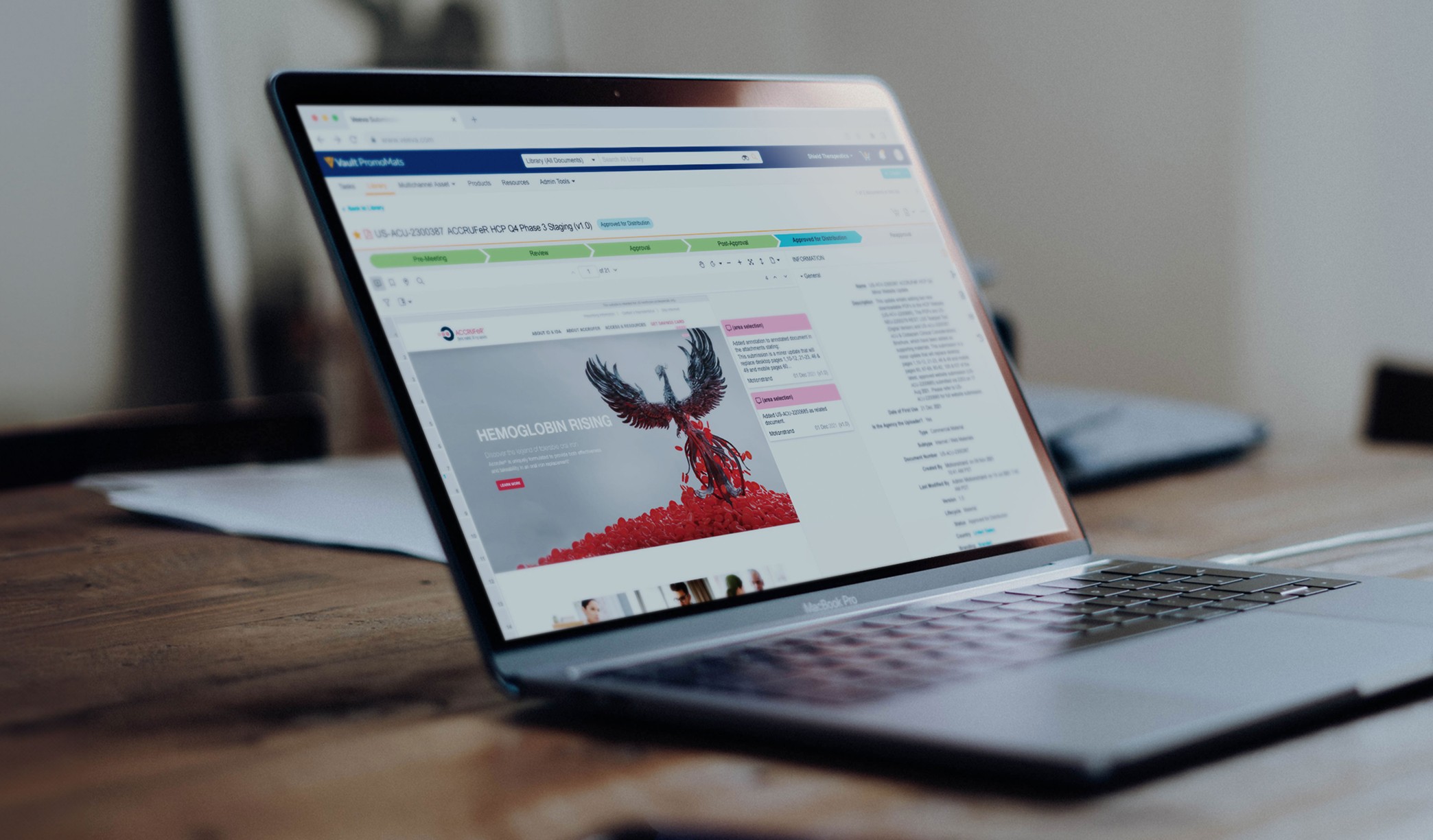
Motionstrand submitted the developed website for PMRC review through Veeva Vault in the final phase. Our team's extensive experience in legal reviews ensured a meticulous submission process, addressing content accuracy, font compliance, and AA ADA accessibility standards. By proactively managing the approval process, we minimized delays and secured a legally sound, launch-ready digital platform for ACCRUFeR®.
Results
Through expert UX, UI, and Drupal development, we streamlined the submission process through Veeva Vault, ensuring a smooth approval and on-time launch. This allowed Shield Therapeutics to focus on market expansion, resulting in higher prescription rates and strong patient adoption, due to its reduced side effects compared to traditional iron supplements. ACCRUFeR® has since experienced steady U.S. sales growth and expanded internationally into Europe, Canada, and China. By delivering a seamless, regulatory-compliant digital platform, Motionstrand helped accelerate ACCRUFeR®’s market impact, supporting its position as a leading treatment for iron deficiency anemia.








